Corporate InformationResearch & Development, Production [Research Introduction]

Research Introduction
Meeting healthcare needs with unique products and technologies
The NIPRO Group has three research and development centers, each respectively focusing on medical devices, pharmaceuticals, and production technology at the Life Science Site in Kusatsu City, Shiga Prefecture.
On the same premises, we have a training facility, NIPRO Institute for Medical Practice (NIPRO iMEP). At NIPRO iMEP, we train healthcare professionals on how to use medical devices, obtain feedback about our products directly from training participants, and promptly reflect their voices in our research and development processes to create new products or improve existing ones.
In December 2016, NIPRO established the Research and Development Center for Regenerative Medicine in Sapporo City and has been conducting research and development of equipment and consumables for the production of regenerative medicine products. Moreover, under a joint research agreement concluded with the University of Tokyo Hospital in March 2019, we established the University of Tokyo and Nipro Research and Development Center at the University of Tokyo Hospital and initiated joint research and development projects with doctors from various specialties to develop next-generation innovative medical technologies and equipment.
The NIPRO Group has been producing creative products that fulfill user needs at these sites.
Research & Development Laboratory

- Development of cellular and regenerative medicine products
- Development of medical devices for hemodialysis
- Development of general medical devices
- Development of products for cardiovascular and interventional applications
- Development of artificial organ-related products
- Development of orthopedic products
- Development of testing and diagnostic reagents
- Development of enzyme products
- Development of functional pharmaceutical containers
- Development of medical glass products
- CR Center
VIEW MORE
Development of cellular and regenerative medicine products
We are proceeding with a comparative study to evaluate the outcomes of the use of Autologous bone-marrow mesenchymal stem cell, which has obtained conditional and time-limited marketing approval as a regenerative medicine product for the treatment of patients with traumatic spinal cord injury, and we are now aiming for obtaining full approval. This product is intended to alleviate neurological symptoms and motor paralysis due to nerve injury by cultivating patients’ autologous mesenchymal stem cells and implanting them through a vein.
Our product development is not limited to regenerative medicine products, but also include peripheral products such as culture media, culture apparatus, cryogenic apparatus, and manufacturing equipment. Through these activities, the company will streamline the production of Autologous bone-marrow mesenchymal stem cell and utilize the experience and knowledge acquired from the project to further develop various types of peripheral products for commercialization.

Autologous bone-marrow mesenchymal stem cell
Automated cell culture systems for ES/iPS cells
Development of medical devices for hemodialysis
We offer comprehensive support for dialysis treatment through the development of a wide range of products, including dialyzers which remove waste products and regulate the water balance in the body, blood tubing sets, single-use needles for dialysis, blood access indwelling catheters, monitoring devices for dialysis, and dialysis-related drugs.
We carry out research and development activities to find solutions for dialysis issues from various perspectives, with the aim of improving the performance and safety of dialysis through research on the structures and materials of hollow fibers, which play a central role in dialyzer performance, and the development of needles with a mechanism to prevent needle stick injury during needle removal.

Dialyzers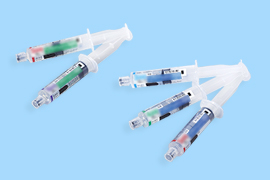
Blood coagulation inhibitors
Single-use needles for dialysis
Development of general medical devices
We develop medical devices related to infusion, catheterization, and infertility treatment as well as disposable medical devices with safety mechanisms.
Our infusion-related products include needleless infusion and sterile infusion systems. Our catheter-related products include implantable infusion ports for long-term use and antithrombotic catheters. Our products for infertility treatment include ultrasonic aspiration biopsy kits and embryo transfer catheters. Our safety-related products include high-spec, value-added devices such as needles with a needle stick injury prevention mechanism that are easy and safe to use.
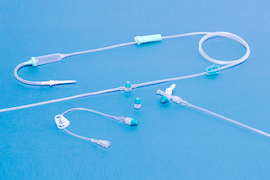
Needleless infusion systems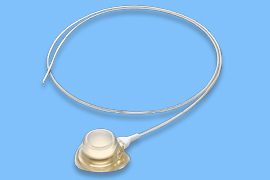
Implantable infusion ports for long-term use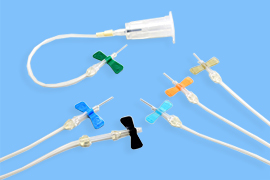
Winged needles with needle stick injury prevention mechanism
Development of products for cardiovascular and interventional applications
We are working to develop and commercialize medical devices for cardiovascular diagnosis and treatment, including PTCA balloon catheters that dilate stenotic coronary arteries by balloon inflation, and thrombus-trapping catheters that trap thrombi and foreign substances that are scattered during procedures.
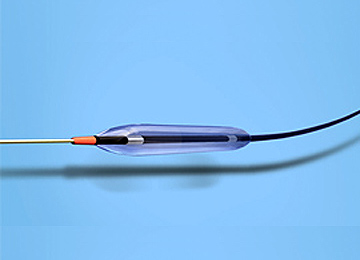
PTCA balloon catheters
Thrombus-trapping catheters
Development of artificial organ-related products
We develop and commercialize oxygenators that act as artificial lungs during cardiac surgery to remove carbon dioxide from and add oxygen to blood, membrane oxygenators with a venous reservoir that filters and collects blood drained from patients, and ventricular assist devices with antithrombotic features, high durability, and high inflow/outflow rates.

Oxygenators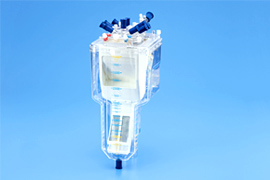
Reservoirs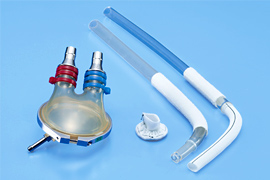
Ventricular assist devices
Development of orthopedic products
We develop implantable medical devices for orthopedic use. These products include bioabsorbable/biodegradable medical devices that are used for patients with peripheral nerve injury to induce regeneration and functional reconstruction of nerves.

Collagen-containing absorbable nerve regeneration inducing materials
Development of testing and diagnostic reagents
We develop new diagnostic agents for early diagnosis and agents to determine therapeutic effects, as well as diagnostic testing systems that can be easily used anywhere. We are also working to develop and commercialize diagnostic systems to detect Alzheimer’s disease, drug-resistant tuberculosis, and COVID-19, as well as systems to monitor blood glucose levels.
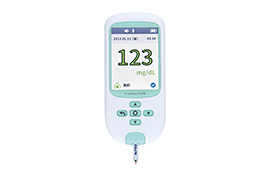
Glucose self-monitoring devices
Diagnostic agents
Diagnostic agents
Development of enzyme products
The main enzyme products we develop are enzyme raw materials for medical use, particularly for laboratory testing. We develop products using heat-resistant enzymes derived from thermophilic fungi, technology for application of these enzymes, genetic recombination technologies, and technologies for producing proteins and substances using microorganisms.
Enzyme products
Development of functional pharmaceutical containers
We develop functional containers that keep stability of drugs and can be used easily and safely. We have commercialized dual chamber bags, dual chamber pre-filled syringes, half-type kits, and preservation-free eyedroppers. Components and manufacturing equipment for containers can also be manufactured within the NIPRO Group. This allows us to design containers while taking into consideration the entire manufacturing processes through to the finished product.
Dual chamber bags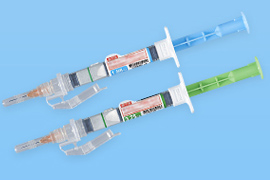
Dual chamber pre-filled syringes
Eyedroppers
Development of medical glass products
We develop medical glass containers with high chemical resistance by removing the degraded inner surfaces of glass products that are caused by molding processing to improve the quality of inner surfaces. We also work to add advanced features to pre-filled syringes that are already washed, arranged in lines, and sterile-packed.

Vials
Pre-filled syringes
CR Center
Once a medical device is designed and developed, it goes through clinical development (such as clinical trials required for regulatory submission), regulatory submission, approval by the Minister of Health, Labour and Welfare, and reimbursement pricing before it becomes available in clinical settings. In addition, we can conduct large-scale surveillance of the use of medical devices after they are launched. At most companies, these activities are conducted by different departments. In contrast, NIPRO has a unique system in which the CR Center takes responsibility for the whole process, including clinical development, regulatory submission, negotiations for reimbursement pricing, and post-marketing surveillance.
Pharmaceutical Research Center

- Development of injectable drugs
- Development of oral drugs
- Development of external use drugs
- Development of anticancer drugs and biosimilars
VIEW MORE
Development of injectable drugs
We actively develop value-added products including user-friendly injection kits and long-acting injections. We also develop pre-mixed IV bag solutions that are already diluted, liquid/powder dual chamber bags, and dual chamber pre-filled syringes.
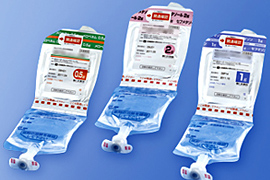
Dual chamber bags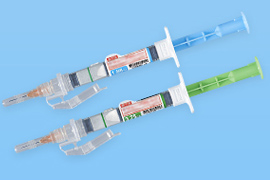
Dual chamber pre-filled syringes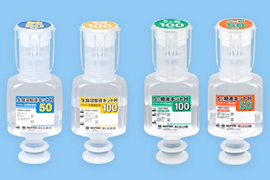
Half-type kits
Development of oral drugs
In addition to common oral drugs, we develop value-added products with unique dosage methods, including orally disintegrating tablets that can be taken without water. Our manufacturing facilities are equipped with a highly-effective containment system that allows us to develop drugs with high pharmacological activity. We also provide drugs with unique labeling and packaging features, such as labeling of substance names on tablets, individual packaging, and aluminum pillow packaging, in order to offer improved convenience for patients and healthcare professionals.
Orally disintegrating tablets (OD tablets)
Orally disintegrating films (OD films)
Half-dose formulations
Development of external use drugs
We develop thin and light tapes and poultices with good adherence and stretching properties, as well as semisolid preparations such as creams that are gentle on the skin, with the aim of launching them outside of Japan as well.
We also develop microneedle patches, which are transdermal preparations created based on the novel idea of attaching an injectable drug on the skin.

Poultices
Tapes
Ointments/Creams
Development of anticancer drugs and biosimilars
Although the markets for anticancer and biological drugs have been growing rapidly, there is a demand for more affordable generic products because these drugs are generally expensive. We actively develop generic products in cooperation with biological API manufacturers that provide products with equivalent quality to brand name drugs at more competitive prices.
Production Technology Center

The Production Technology Center is responsible for the development of production technologies used across the NIPRO Group. It designs production equipment and facilities that fulfill all of the needs of the NIPRO Group’s various plants.
It also develops specific system design technologies, such as electric systems, circuits, and programs to help increase productivity across the NIPRO Group.
- Designing and building equipment and facilities
- Inspection, analysis, and maintenance of equipment and facilities
- Development of production technologies
- Designing and building electric systems, circuits, and programs
- Inspection, analysis, and maintenance of electric systems, circuits, and programs
Images of product names are blurred because advertisement of medical devices and pharmaceuticals targeting lay persons is restricted by the Act on Securing Quality, Efficacy and Safety of Products Including Pharmaceuticals and Medical Devices.